ARTICLE SUMMARY:
In 1985, a first-of-its-kind intragastric balloon device debuted on the US market, as a preliminary, temporary intervention prior to invasive bariatric surgery for the treatment of a booming global epidemic: obesity.
Thirty-five years ago, in 1985, US President Ronald Reagan was sworn in for his second term in office, and the FDA approved the first blood test for AIDS (enzyme-linked immunosorbent assay, or  ELISA), which has subsequently been used to screen all blood donations in the US. Also in this year, the first domain name was registered—Symbolics.com—by computer systems firm Symbolics Inc. Back then, “.edu” domain names predominated over commercial “.com” domains. (Today there are more than 350 million domain names registered, dominated by dot-coms).
ELISA), which has subsequently been used to screen all blood donations in the US. Also in this year, the first domain name was registered—Symbolics.com—by computer systems firm Symbolics Inc. Back then, “.edu” domain names predominated over commercial “.com” domains. (Today there are more than 350 million domain names registered, dominated by dot-coms).
 Also in this year, the wreck of the RMS Titanic was located by a joint American-French expedition, following its sinking 73 years prior in 1912, making headlines around the world. The wreck was found in two main pieces lying in a two-square-mile debris field at a depth of about 12,500 feet (2.37 miles), about 370 miles south-southeast off the coast of Newfoundland. Many elaborate plans were devised over the years to raise it from the ocean floor, but the decaying wreck was deemed too fragile, and is now protected under the 2001 United Nations Educational, Scientific and Cultural Organization (UNESCO) Convention on the Protection of Underwater Cultural Heritage.
Also in this year, the wreck of the RMS Titanic was located by a joint American-French expedition, following its sinking 73 years prior in 1912, making headlines around the world. The wreck was found in two main pieces lying in a two-square-mile debris field at a depth of about 12,500 feet (2.37 miles), about 370 miles south-southeast off the coast of Newfoundland. Many elaborate plans were devised over the years to raise it from the ocean floor, but the decaying wreck was deemed too fragile, and is now protected under the 2001 United Nations Educational, Scientific and Cultural Organization (UNESCO) Convention on the Protection of Underwater Cultural Heritage.
Also in 1985, a first-of-its-kind medical device debuted on the US market, as a preliminary, temporary intervention prior to invasive bariatric surgery, for the treatment of a booming global epidemic: obesity. The polyurethane Garren-Edwards Bubble intragastric balloon (IGB) from American Edwards Laboratories (parent company Baxter-Travenol Laboratories) was indicated for temporary use as a weight loss device in people who are at least 20% over their ideal weight who had failed behavior modification therapy or dietary management. In terms of the mechanism of action, the device occupied space in the stomach in order to induce early satiety, and delay gastric emptying, leading to smaller meals and subsequent weight loss. The FDA based its approval on clinical studies of 78 overweight patients, in which 41 patients treated for six months lost an average of 40 pounds.
Obesity and overweight is a complex, multifactorial, and largely preventable disease, affecting over a third of the world’s population. And, its prevalence has skyrocketed even after the first IGB entered the US market in 1985. As of that year, no US state had an obesity rate higher than 15%, but as of 2017, seven states (West Virginia, Mississippi, Oklahoma, Iowa, Alabama, Louisiana and Arkansas) had rates of 35-38%, according to the Centers for Disease Control and Prevention. (As an aside, Colorado ranks the lowest in the US, at 22.6%.) If trends continue, by 2030 an estimated 38% of the world’s adult population will be overweight and another 20% will be obese.
In a Garren-Edwards Bubble implantation procedure, the patient was sedated and a hollow plastic cylinder was inserted through the mouth in its deflated form, while a gastroenterologist used an endoscope to see into the stomach to ensure that the device is properly placed. The bubble was then inflated until it was about two inches in diameter and about 3.5 inches long (see illustration below). An endoscope was also used to remove the balloon, which was deflated by puncturing and then pulled out through the mouth.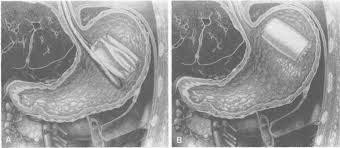
However, excitement over the novel balloon device started to wane in November 1986, when American Edwards warned doctors to curtail use of its IGB because the device has been associated with one patient death and about 80 serious medical complications, after about 17,000 devices had been inserted nationwide. New labeling information mailed to the 2,000 gastroenterologists trained to insert the device warned of new and more severe side effects than previously reported, recommended removal of the balloon after three months (four months was previously recommended), and limited its use to only the most severely overweight patients.
Manufacture of the device was discontinued in 1988, and approval was withdrawn in 1992 because of significant patient complications such as perforations and ulcers of the stomach, and intestinal blockages, as well as limited and recidivistic weight loss.
Since then, in an effort to address the huge and growing unmet clinical need for a bariatric surgery alternative, device manufacturers have continued to improve balloon system design, and address complication and long-term weight loss concerns. In August 2015, the ReShape Duo Integrated Dual Balloon System from ReShape Medical received FDA PMA approval (company acquired by EnteroMedics in October 2017 in a $38 million cash-and-stock deal). Other IGBs on the market include the ORBERA Intragastric Balloon from Apollo Endosurgery (also FDA PMA-approved in August 2015), and the Obalon Balloon System from Obalon Therapeutics (FDA PMA-approved in September 2016). Clinical data continues to be gathered on these marketed devices, and a variety of balloons are available outside the US. More than a dozen IGBs are under clinical investigation around the world. (See our MedTech Strategist archives, including the articles listed on the right side of this page, for more information on this growing space.)
Importantly also, investors are placing bets on the IGB market. Last month, Allurion Technologies, which markets an IGB outside the US, raised $34 million in funding through a securities financing and a growth capital term loan. The round was led by Novalis Lifesciences and Romulus Capital, with participation from Ido Investments and ex-Covidien CEO Jose Almeida. It also includes a growth capital term loan from Bridge Bank. The Natick, MA-based company has developed the Elipse Balloon and a Bluetooth body composition scale that pairs with its smartphone app. The balloon is designed to be placed and removed without surgery, endoscopy or anesthesia. It is swallowed in a capsule during an outpatient office visit and is designed to remain in the stomach for about four months, after which it opens and passes naturally from the body. More than 20,000 devices have been distributed in 25 countries.
#bariatricsurgery #bariatric #IGB #intragastricballoon #obesity #gastrointestinal #gastric #ulcer #metabolic #CDC #RonaldReagan #AIDS #ELISA #Titanic #UNESCO #GarrenEdwardsBubble #AmericanEdwardsLaboratories #BaxterTravenol #ReshapeMedical #ReShapeDuo #Enteromedics #ApolloEndosurgery #Orbera #Obalon #ObalonTherapeutics #AllurionTechnologies #JoseAlmeida #Covidien #innovation #ThisWeekinMedtechHistory #InMedTechHistory #IMH #medicaldevice #medtech #CommunityBlog #MedTechStrategist #TracySchaaf
![]() Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
