ARTICLE SUMMARY:
Jupiter Endovascular is running a US pivotal trial on a transcatheter device that can transform along its entire length from flexible for navigation to firm as a base of operation for therapy delivery. Its first application is the treatment of pulmonary embolism, an underpenetrated $3 billion market.
Jupiter Endovascular recently secured $21 million in funding to support the pivotal trial of its new device for treating pulmonary embolism, the third leading cause of cardiovascular-related deaths, following heart attacks and strokes.
This life-threatening condition occurs when a blood clot in the pulmonary artery obstructs blood flow from the heart to the lungs, and it has seen significant innovation in recent years, largely due to the success of Inari Medical, which had a strong initial public offering supported by its portfolio of devices specifically designed for venous system clots.
Historically, interventionalists had to rely on coronary devices repurposed for use in veins, even though the two types of vasculatures have different requirements. Currently, at least 24 companies are developing thrombectomy platforms for pulmonary embolism, ranging from market-ready devices to those still in development.
What sets Jupiter Endovascular apart from its competitors is its focus on a specific problem in PE treatment that has been largely overlooked, which contributes to the underpenetration of existing devices in the market, procedural complications, and limited efficacy in treatment. The large bore size and stiffness of current commercial catheters risk vessel and heart trauma. This commonly results in cases ending prematurely with significant clot burden remaining, particularly in the distal pulmonary arteries, which leaves patients with residual disease that can affect their long-term quality of life.
Alex Tilson, the inventor of the company’s Endoportal Control platform technology, and the founder of Neptune Medical, from which Jupiter Endovascular spun off to focus on cardiovascular applications of the technology, set out to create a transcatheter device with unlimited properties in terms of flexibility and 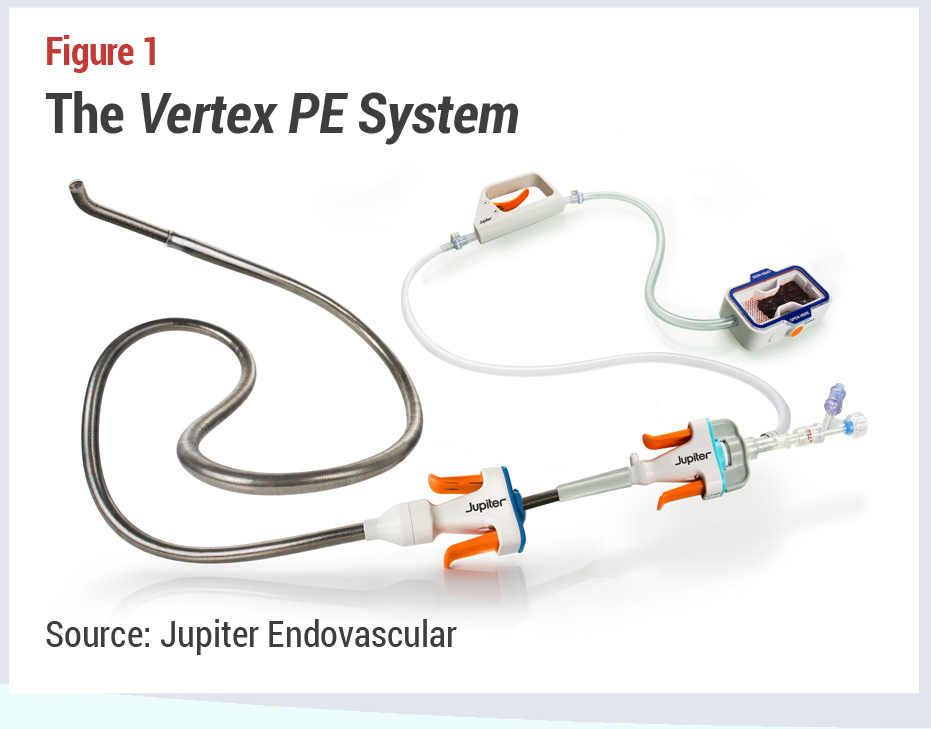 support. Jupiter CEO Carl St. Bernard explains, “Interventional medicine today is generally a balancing act between flexibility for access,” and, once at the destination for treatment, “support so you can actually deliver a therapy.” Interventionalists constantly change wires and catheters during procedures to find the optimal way to treat patients. This movement of multiple stiff devices through the right heart creates a risk of decompensation for patients, he adds.
support. Jupiter CEO Carl St. Bernard explains, “Interventional medicine today is generally a balancing act between flexibility for access,” and, once at the destination for treatment, “support so you can actually deliver a therapy.” Interventionalists constantly change wires and catheters during procedures to find the optimal way to treat patients. This movement of multiple stiff devices through the right heart creates a risk of decompensation for patients, he adds.
Jupiter Endovascular’s Vertex Pulmonary Embolectomy System is flexible enough to track over a soft guidewire,which enables gentle navigation through the right heart and pulmonary arteries. Once at the clot,the operator simply presses a handpiece to firm the entire length of the device using hydrostatic pressure while conforming to the anatomy. The fixed catheter then serves as a stable base to enable aspiration deep in the pulmonary arteries (see Figure 1). This is a manual, rather than robotic technology, St. Bernard clarifies. “It’s almost like having a guidewire that you can move anywhere you want to go, and once there, you can lock it into the right shape to give optimal support for therapy.”
The company’s Endoportal Control platform has many potential applications. For its initial cardiovascular application, the spinout chose pulmonary embolism as an established market that still represents an unmet clinical need. For starters, despite billions of dollars invested and the presence of major strategics in the space (Boston Scientific, for example) as well as mid-cap leaders Inari and Penumbra Medical, the market is only about 20-30% penetrated, while utilization is concentrated within about 200 hospitals. St. Bernard recalls, “We had to ask ourselves why, with a great innovation that saves lives, it was not being accessed by the majority of the eligible patients that could benefit from it.”
The answer has two parts, the CEO notes: how to get to the clot and what to do when you get there. As to the former challenge, “Putting a 20-plus French catheter through the center of someone’s heart today, through their pulmonary valves and into their pulmonary arteries is a torturous journey for that catheter and for that patient”; it causes cardiac stress and bears a non-negligible risk of cardiac collapse.
Further, because it is challenging to navigate to the precise spot of treatment, many patients don’t get complete treatment, in terms of eradicating the whole clot and any distal fragments. Finally, “Many doctors are anxious about doing this challenging procedure because of potential safety issues.”
How the device gets to the clot can influence the choice of tool for removing it. St. Bernard says, “Our system was designed to get to the clot safely with less trauma to the patient. Physicians tell us that when they can position the catheter right on the blood clot and keep it there as long as they need it, suction works fine in the acute and subacute phases nine out of 10 times. We think we have an opportunity to change how physicians view the procedure.”
Proving the Hypothesis
Preclinical animal labs suggested that the Vertex PE catheter is easily placed and successfully stabilized at the clot. Animal labs and early experience from the first-in-human study SPIRARE I,currently enrolling up to 10 patients in Europe at two sites,suggest that subjects enjoy better hemodynamics because navigating a conventional catheter stretches the heart. “Imagine putting a large, stiff catheter through two chambers of the heart, two valves, and then trying to bend it into a pulmonary artery. The stiff catheter always wants to go straight, and that distorts the chambers of the heart of a patient who is already under duress,” St. Bernard points out. By contrast, the interventionalist can precisely control the Vertex PE catheter. “It stays right in the center of the artery.”
He suggests that with a potential safety advantage, clinicians might become more comfortable navigating further into the arterial tree where distal clot fragments tend to lodge. In early human studies there is a suggestion that “when physicians have more control and the confidence that they’re not injuring patients, they are comfortable delivering aspiration deeper into various pulmonary arteries, where in many cases, they wouldn’t have made as many passes because they were worried about cardiovascular injury and cardiovascular collapse.”
St. Bernard notes that the company will also be monitoring the amount of clot the Vertex PE catheter can remove (by aspiration) in one go. “In our early human cases, we are seeing that operators may be removing more than they can with commercial technology.” That remains to be seen from ongoing clinical studies.
Jupiter Endovascular is in the process of validating these advantages in SPIRARE I and its US trial, SPIRARE II, aprospective, single-arm, multicenter pivotal trial that will include up to 145 patients with acute, intermediate-risk PE treated with the Vertex PE System at up to 25 sites. Investigators will evaluate both procedural and clinical benefits across measures of safety, right heart function, and clinical improvement from the initiation of the procedure to 30 days postprocedure.
In late October, the company reported the success of the first clinical case in the US. Mitchell Weinberg, MD, chair of cardiology at Staten Island University Hospital, Northwell Health reported “an excellent procedural result with remarkable speed and ease, especially given that it was our first time using this technology.” Operating at the same institution, Vincent Gallo, MD, director of vascular and interventional radiology, described the procedure’s advantages over the standard of care that requires multiple guidewires and ancillary devices to reach the target vessels. He said, “We eliminated many of these extra steps and device exchanges, resulting in a much simpler procedure that allowed us to focus less on gaining vessel access and more on treating the patient.”
As a newcomer to a crowded space, these are the ways that Jupiter Endovascular plans to demonstrate competitive advantages: by making the procedure safer for the patient, faster and more productive than existing devices, and perhaps enabling a greater reach into the distal pulmonary arteries. “We are closely looking at mean pulmonary artery pressure at the start of the case and after the physician is done, to understand if we can significantly reduce that contributor to right heart failure. That would be a big win for us,” says St. Bernard.
Jupiter Endovascular is fundamentally differentiated. “We are the only company really concerned about the journey to the clot,” he adds.

