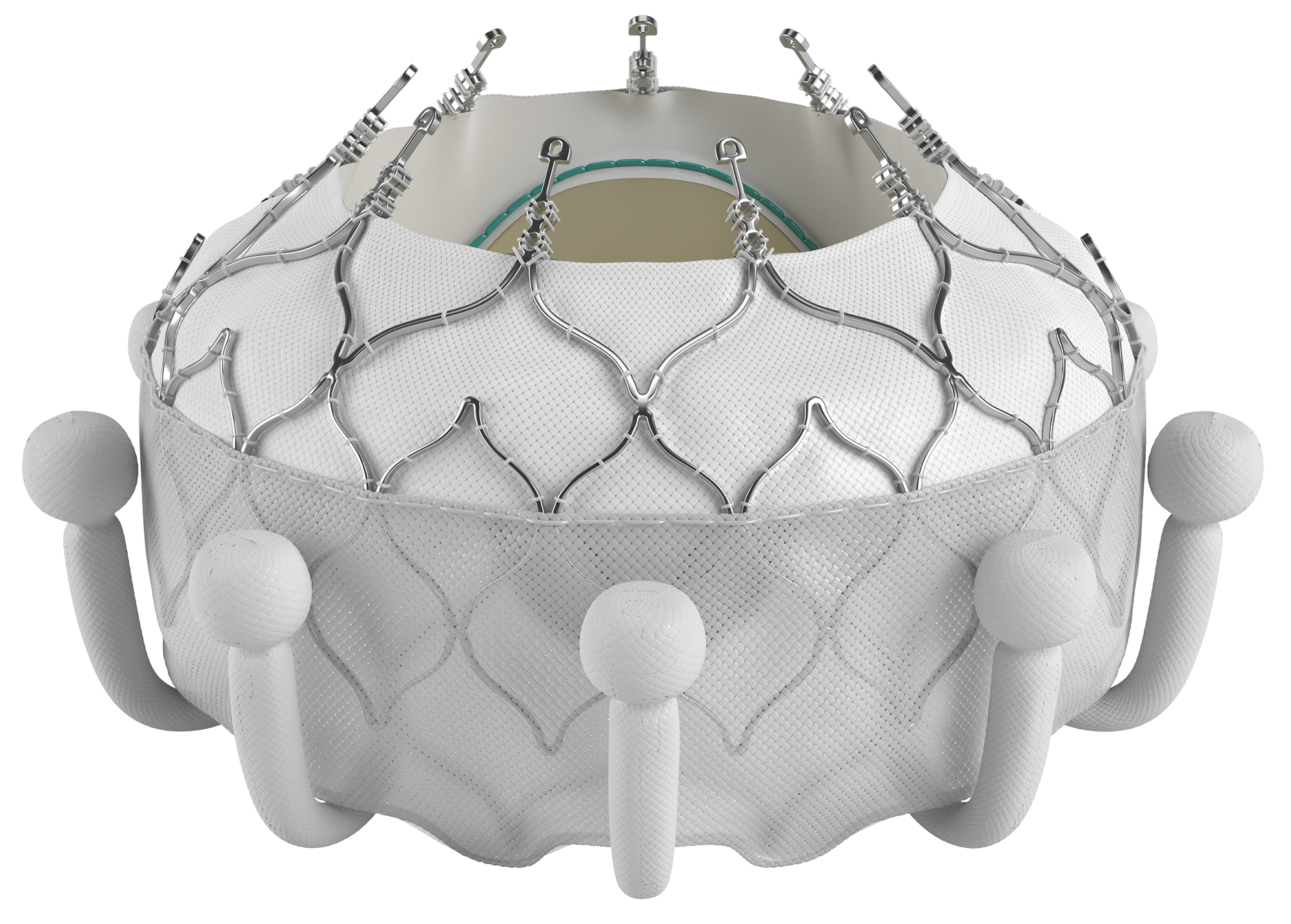
ARTICLE SUMMARY:
Novel transcatheter tricuspid valve devices from Abbott Laboratories and Edwards Lifesciences have been enrolled as pilot cases in CMS’ new Transitional Coverage for Emerging Technologies (TCET) pathway.
CMS’ recently launched Transitional Coverage for Emerging Technologies (TCET) pathway appears to be getting its first tryouts with new transcatheter tricuspid valve devices.
The Medicare agency finalized TCET in August of this year as a mechanism to streamline evidence review and coverage determinations for select FDA Breakthrough Devices, via an enhanced coverage review process designed to start prior to FDA authorization. Since then, CMS has officially marked two pending coverage analyses as pilot runs for TCET.