ARTICLE SUMMARY:
Four hot spots in policy docs this summer from the EU, FDA, CMS, and China.
Docs of the Month is a regular column highlighting key findings from Pathways’ Document Depot, a database of global medtech regulatory and policy official documents, including rules, guidance documents, memos, white papers, and more from national authorities, non-governmental groups, and global organizations.
Operations tend to slow down over the summer months, but there’s still a lot happening at the government agency level.
Pathways’ Document Depot captured about 80 medtech policy documents published from June 20 (the official start of summer in the Northern Hemisphere) to August 20, including many with significant impacts on how medtech firms do business.
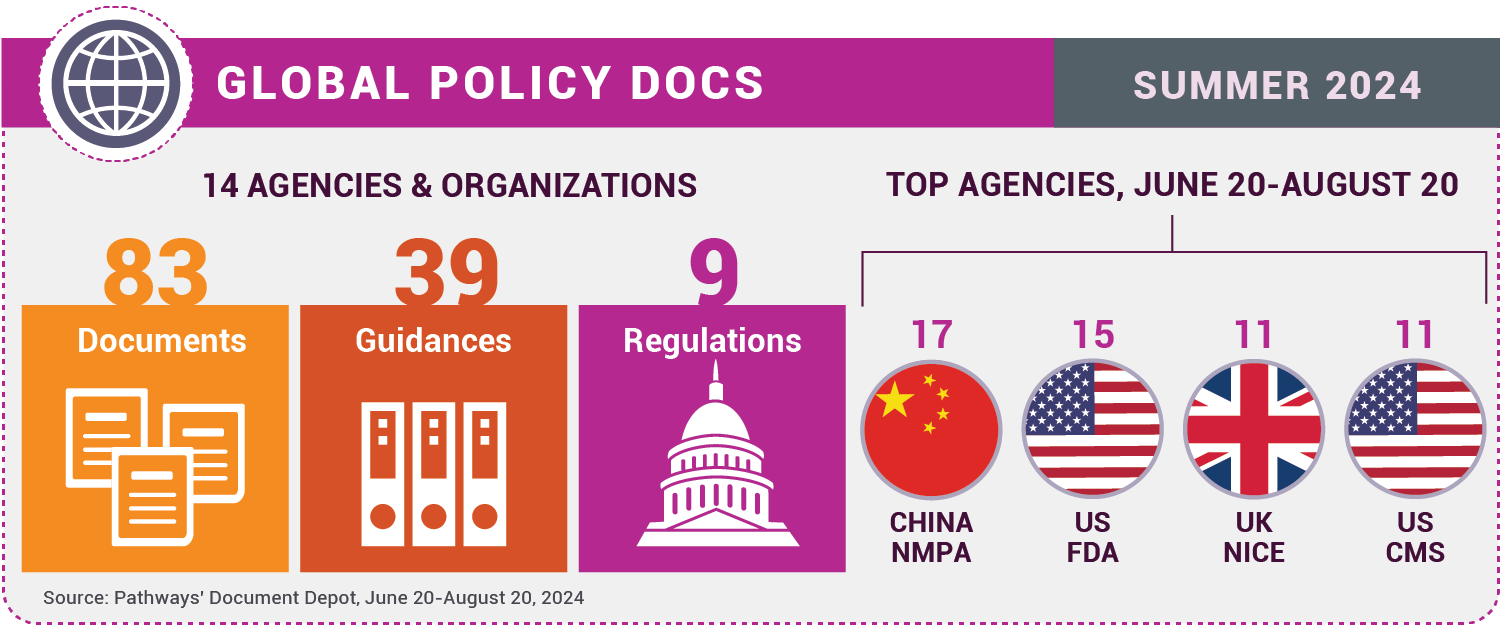
Here’s a spotlight on noteworthy items published by four agencies from around the world during the period.

