ARTICLE SUMMARY:
Genome editing using the powerful CRISPR tool had its beginnings in 1987, in a lab experiment focused on microbes. This DNA-altering technology now holds enormous but controversial potential for treating and curing diseases caused by genetic mutations, and detecting infectious diseases like COVID-19.
In 1987, the Dow Jones Industrial Average closed above 2,000 for the first time, gaining 8.30 to close at 2,002.25 on January 8 (fast forward 33 years: the DJIA’s high peaked at 29,551.42 on February 12, 2020). On June 12 of that year, during a visit to Berlin, Germany, US President Ronald Reagan famously challenged Soviet Premier Mikhail Gorbachev to tear down the Berlin Wall. Also in 1987, the antidepressant drug Prozac made its US debut, the song “Walk Like an Egyptian” by The Bangles topped the Billboard music charts, and Best Picture at the 60th Academy Awards went to The Last Emperor.
high peaked at 29,551.42 on February 12, 2020). On June 12 of that year, during a visit to Berlin, Germany, US President Ronald Reagan famously challenged Soviet Premier Mikhail Gorbachev to tear down the Berlin Wall. Also in 1987, the antidepressant drug Prozac made its US debut, the song “Walk Like an Egyptian” by The Bangles topped the Billboard music charts, and Best Picture at the 60th Academy Awards went to The Last Emperor.
 Also in 1987, in a lab at Osaka University in Japan, an unusual repetitive DNA sequence in the Escherichia coli (E. coli) genome was discovered, that would lead more than 30 years later into the development of a simple yet powerful genome engineering tool called CRISPR (clustered regularly interspaced short palindromic repeats). Today, CRISPR is poised to revolutionize the field of biology, as it allows researchers using “molecular scissors” to easily alter DNA sequences and modify gene function, with many potential (and some controversial) applications include correcting genetic defects, treating and preventing the spread of diseases, and in agriculture to improve crop yields, drought tolerance, and nutritional properties.
Also in 1987, in a lab at Osaka University in Japan, an unusual repetitive DNA sequence in the Escherichia coli (E. coli) genome was discovered, that would lead more than 30 years later into the development of a simple yet powerful genome engineering tool called CRISPR (clustered regularly interspaced short palindromic repeats). Today, CRISPR is poised to revolutionize the field of biology, as it allows researchers using “molecular scissors” to easily alter DNA sequences and modify gene function, with many potential (and some controversial) applications include correcting genetic defects, treating and preventing the spread of diseases, and in agriculture to improve crop yields, drought tolerance, and nutritional properties.
In that year, Japanese molecular biologist Yoshizumi Ishino and his research team detected the first CRISPRs while analyzing the iap gene in the gut microbe E.coli. To better understand how the gene worked, the scientists also sequenced some of the DNA surrounding it. Near the iap gene lay five identical segments of DNA, according to a February 2015 article in Quanta magazine. DNA is made up of building blocks called bases, and the five segments were each composed of the same 29 bases. These repeat sequences were separated from each other by 32-base blocks of DNA, called spacers. Unlike the repeat sequences, each of the spacers had a unique sequence. At the time, DNA sequencing hadn’t yet been invented, and so the researchers weren’t able to determine the biological function of these mysterious repeated sequences.
of DNA, according to a February 2015 article in Quanta magazine. DNA is made up of building blocks called bases, and the five segments were each composed of the same 29 bases. These repeat sequences were separated from each other by 32-base blocks of DNA, called spacers. Unlike the repeat sequences, each of the spacers had a unique sequence. At the time, DNA sequencing hadn’t yet been invented, and so the researchers weren’t able to determine the biological function of these mysterious repeated sequences.
Fast-forward to the 1990s, when rapid genetic sequencing became possible. At that time, the repeated segments of genetic material began to be discovered in a wide range of microbe species. In 2001, researchers Francisco Mojica and Ruud Jansen coined the name CRISPR for these repeating sections. In 2007, it was discovered that these clustered repeat sequences operated as a safeguard against bacteriophages. Researchers realized that bacteria was incorporating segments of DNA from viral invaders into its own genome and using it as an early warning system against attacks by the same virus, according to a July 2019 Brainfacts.org article. Particular enzymes, including one called Cas9, serve as weapons against the invaders. Cas9 carries a copy of the CRISPR sequence with it, searching for matching DNA in the viruses. Wherever it finds that matching DNA, Cas9 severs it and breaks it apart. Together, they form the CRISPR-Cas9 system. From this important research it was shown that microbes in nature have been using CRISPR molecules as part of a sophisticated immune system to edit their own DNA for millions of years.
In 2012, researchers Jennifer Doudna, Emmanuelle Charpentier, and a separate team led by Lithuanian biochemist Virginijus Siksnys brought this science to a whole new level, when they discovered that Cas9 could be reprogrammed to target, destroy, or replace specific genetic sequences, and not just in bacteria and viruses. That’s when the potential for editing genes really became a reality, according to the Brainfacts.org article.
Thanks to CRISPR, the field of genome editing is now evolving rapidly, with the potential to dramatically impact human disease. It is being explored in early-stage human research on a wide variety of diseases, including single-gene disorders such as cystic fibrosis, hemophilia, and sickle cell disease—in the latter, there have been early and positive results. In fact, there are more than 3,000 genetic diseases, such as color blindness and Huntington’s disease, caused by one wrongly placed nucleotide in the DNA. The technology also holds promise for the treatment and prevention of complex diseases such as cancer, heart disease, mental illness, and human immunodeficiency virus (HIV) infection. However, CRISPR is not without controversy, as scientists and medical experts debate the ethical implications of editing the genomes of human embryos to permanently alter the human species.
This March, CRISPR was administered directly into a human body for the first time, to treat a patient with the hereditary blindness disorder Leber’s congenital amaurosis 10. In this treatment, doctors at the Casey Eye Institute in Portland, OR, injected microscopic droplets carrying a harmless virus that had been engineered to deliver the instructions to manufacture the CRISPR gene-editing machinery. The goal of the treatment is that once the virus carrying the CRISPR instructions has been infused into the eye, the gene-editing tool will delete a mutation in the gene CEP290, that is causing the blindness. That would, researchers hope, restore production of a crucial protein and prevent the death of cells in the retina, as well as revive other cells—enabling patients to regain at least some vision. The landmark BRILLIANCE trial, which will eventually involve a total of 18 patients, is being sponsored by Editas Medicine and Allergan plc.
In addition to being able to repair DNA, CRISPR is now also being used as a low-cost diagnostic tool to detect infectious diseases. In May of this year, Sherlock Biosciences became the first company to receive FDA authorization for a CRISPR technology, when it was granted an emergency use authorization (EUA) from the FDA for its COVID-19 diagnostic assay. The CRISPR SARS-CoV-2 rapid test kit, which works by programming a CRISPR molecule to pick up the novel coronavirus' genetic signature, capitalizes on a CRISPR-based technology developed in the lab of pioneering CRISPR scientist Feng Zhang, from the Broad Institute of MIT and Harvard, and a co-founder of Sherlock Biosciences. The test, which can deliver results in about an hour, is designed for use in high-throughput labs (also see the July 2020 MedTech Strategist article on Sherlock Biosciences, referenced on this page).
Reference for image at the top of this page:
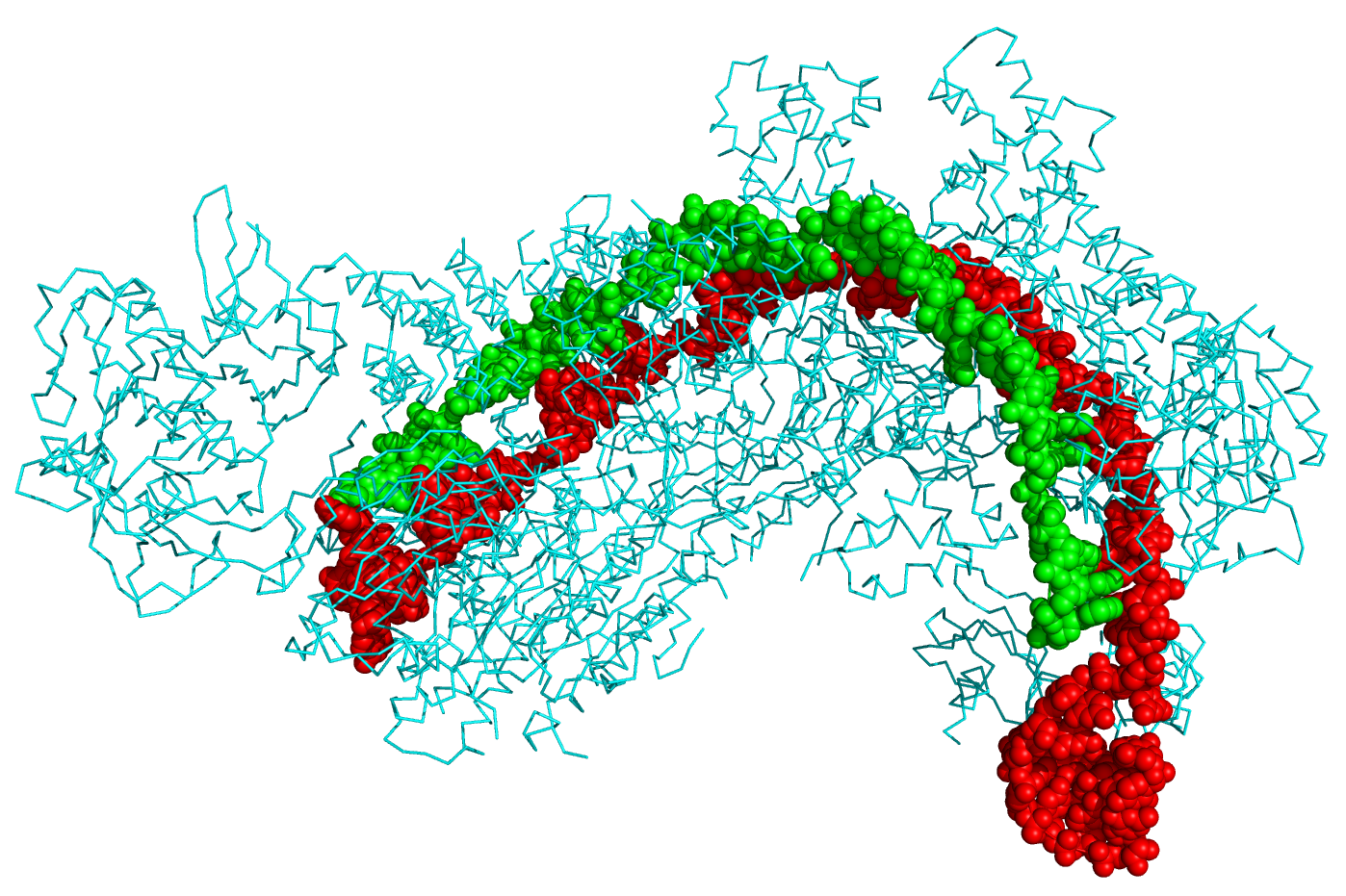
Crystal structure of a CRISPR RNA-guided surveillance complex, Cascade, bound to a ssDNA target. CRISPR system Cascade protein subunits CasA, CasB, CasC, CasD, and CasE (cyan) bound to CRISPR RNA (green) and viral DNA (red) based on PDB 4QYZ and rendered with PyMOL. Source: Wikipedia
![]() Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.