ARTICLE SUMMARY:
Obstructive sleep apnea is the perfect market for medtech innovation: a successful first mover in Inspire Medical, a validated mechanism of action, and a significantly undertreated patient population. Emerging companies are poised to capitalize on the opportunity. Excerpted from our recent feature, Wake Up Call for Sleep Apnea Devices.
Inspire Medical is a shining example of medtech’s promise, for patients and investors. Spun off from Medtronic in 2007 to develop a novel implantable neurostimulation therapy for obstructive sleep apnea, the company went public in 2018 and today has a market cap of just over $6 billion.
The company has innovated a surgically implanted therapy that, guided by breathing patterns, delivers neurostimulation to the hypoglossal nerve (HN) to cause the tongue to move forward and unblock a sleeping OSA patient’s obstructed airway. Creating a market from scratch, Inspire had to demonstrate the clinical efficacy of a novel therapy and establish surgical referral pathways to new treating clinicians. Inspire has also paved the way for reimbursement, which it has successfully gained in all 50 states.
To date, 85,000 patients have been treated with the Inspire hypoglossal nerve stimulation (HNS) platform. With a $10 billion market to itself, Inspire has enjoyed a compound annual growth rate of 65% for the past five years. Recently announced 2024 revenues of about $803 million represent 28% year-on-year growth.
Inspire was the first company to offer an alternative to the prevailing therapy for obstructive sleep apnea: positive airway pressure (more commonly referred to as CPAP for continuous positive airway pressure, although today there are alternative paradigms in the delivery of positive airway pressure). Involving a blower, a hose, and a face mask, air is blown into the patient’s airway during sleep to create a level of pressure that keeps their airway unblocked. CPAP is noninvasive, but it is certainly not unobtrusive, and 50% of patients either can’t tolerate it or don’t choose it. Among CPAP users, compliance is in the 35-50% range, which is another reason why additional therapies are needed for these patients.
That is the unmet medical need around which Inspire has built a new device market, and the need doesn’t end there. The population eligible for treatment is still remarkably unpenetrated. Considering 24 million people in the US with moderate to 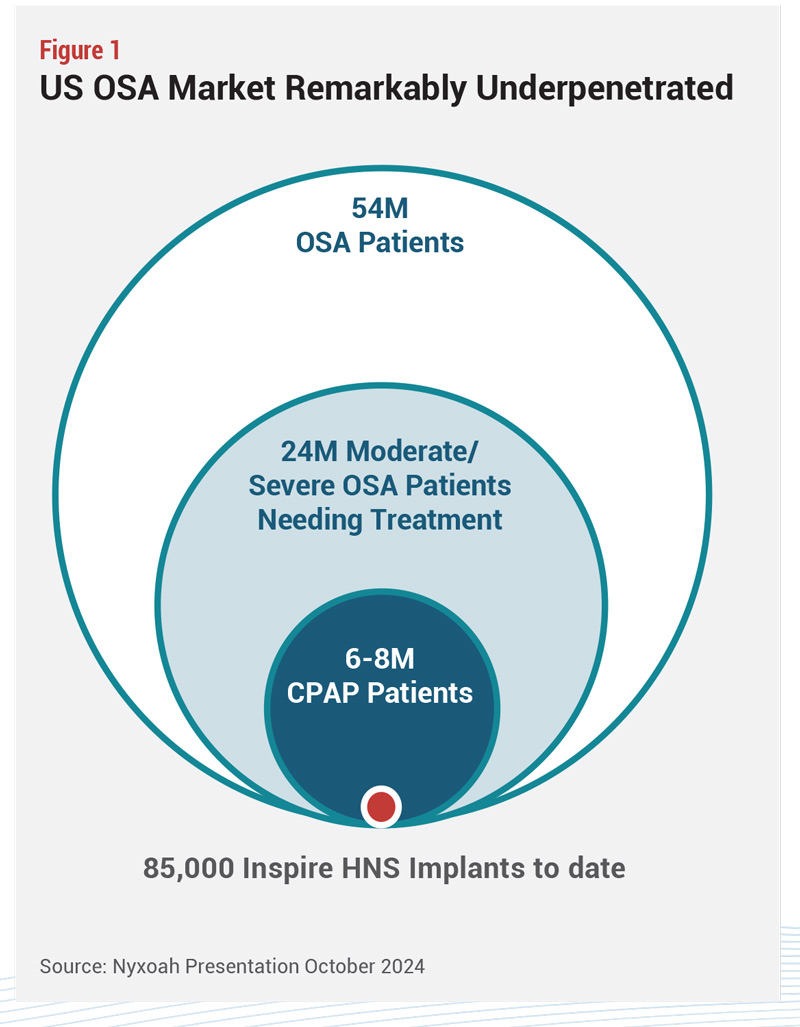 severe OSA, 6 to 8 million on CPAP, and 85,000 treated by HNS, there is plenty of room for growth (see Figure 1). Noninvasive CPAP will probably always be the first-line treatment (in order to be reimbursed for any other therapy, patients must demonstrate that they have failed or cannot tolerate CPAP) but given the sheer number of patients who won’t or can’t use it, there is space for many therapy developers.
severe OSA, 6 to 8 million on CPAP, and 85,000 treated by HNS, there is plenty of room for growth (see Figure 1). Noninvasive CPAP will probably always be the first-line treatment (in order to be reimbursed for any other therapy, patients must demonstrate that they have failed or cannot tolerate CPAP) but given the sheer number of patients who won’t or can’t use it, there is space for many therapy developers.
Inspire’s success has naturally attracted many followers, and today, there are at least six companies iterating around hypoglossal nerve stimulation, with a goal of making the therapy less invasive and more patient friendly. These include, in addition to Inspire Medical, LivaNova, Nyxoah, Restera Sleep, StimAire, and XII Medical. Many are raising significant venture capital rounds. XII Medical, for example, which is still being stealthy, raised a $45 million Series B round in August 2024. (The roman numeral represents the 12th cranial nerve, i.e., the hypoglossal nerve.) Omega Funds led the financing, and Intuitive Ventures joined existing investors Ajax Health, Longview Ventures, Aperture Venture Partners, JobsOhio Growth Capital Fund, an undisclosed strategic investor, and the Cleveland Clinic. The latter founded, incubated, and seeded the start-up, based on work by surgeon and inventor Frank Papay, MD.
In an interview, XII Medical CEO Garrett Schwab was not ready to be more specific, only saying that Chief Technology Officer Anthony Caparso is a serial innovator with deep domain expertise in neuromodulation, and “for physicians, the system is designed to enable a streamlined and straightforward implant procedure and therapy optimization workflow, thereby enabling them to offer treatment to a larger number of patients.” Patients will benefit from reduced surgical invasiveness and a requirement for minimal interactions with the therapy, which “will fit seamlessly into their established daily routines to make it attractive to a wider range of patients and to drive enhanced compliance and utilization.”
Continue reading this feature here.
